Sources of Cyanide
INTRODUCTION
Cyanide is a chemical compound possessing highly
toxic potential. It is present in three spheres that are atmosphere (air),
hydrosphere (water) and in lithosphere (soil). Cyanide is a unique chemical
compound used to manufacture hundreds of everyday goods, and chances are that
today you will use many products that included cyanide in its manufacture.
These products may include vitamins (B12), jewellery, adhesives, computer
electronics, fire retardants, airplane brakes, cosmetics, dyes, nylon, nail
polish remover, paints, pharmaceuticals, Plexiglas, rocket propellant, and
table salt. The manufacturing of these products takes place every day around
the world with little knowledge from the general public that cyanide is a
critical ingredient in their manufacture. Indeed, without cyanide it would not
be possible to manufacture such widely used items as nylon and many vitamins
and other medications.
Products made by using Cyanide
Cyanide is a relatively toxic compound but has been safely
used for well over a hundred years around the world. Many people have a natural
fear of cyanide that arises from a general understanding of its toxicity but a
less thorough understanding of its actual properties and usefulness in our
everyday lives. As with any chemical, cyanide must be properly and safely
handled to avoid harm to people or the environment. The benefit people derive
from cyanide and its many products far outweigh the risk posed to people and
the environment.
The human body has a natural ability to detoxify
small quantities of cyanide, and there is normally a small amount of cyanide
and its breakdown products in the body as a result of everyday activities.
These activities may include the metabolism of vitamin B12, eating of foods
naturally containing cyanide (for example, almonds, Lima beans, coffee and
table salt), exposure to automobile exhaust and smoking cigarettes. In some
form, we are exposed to low levels of natural and man-made cyanide every day without
risk to our health or the environment.
WHAT
IS CYANIDE?
Cyanide is a general term for a group of chemicals
containing carbon (C) and nitrogen (N). The term cyanide in toxicological
profile means a compound that contains the cyanogen (CN) radical (A triple-bonded molecule with a negative one
charge consisting of one atom of carbon in the +2 oxidation state and one atom
of nitrogen in the -3 oxidation state). Since the CN portion of the
compound is of concern in poisons. Cyanide compounds include both naturally
occurring and human-made chemicals. Naturally, cyanide can be produced by
certain bacteria, fungi, algae, and it is found in a number of foods and
plants. The principal human-made cyanide forms are hydrogen cyanide (HCN),
sodium cyanide (NaCN) and potassium cyanide (KCN). Hydrogen cyanide is a
colourless gas with a faint, bitter, almond-like odour. Cyanide is acute toxic
and is lethal if ingested or inhaled.
Cyanide molecule
Cyanide chemical formula
TYPES; HOW IT IS USED?
Cyanide combines with many organic and inorganic
compounds. Because of its unique properties, cyanide is used in the manufacture
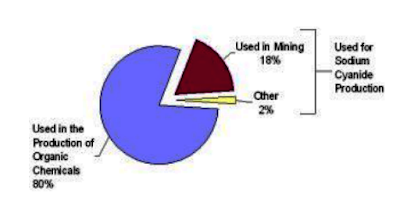
of metal parts and numerous common organic products. About 1.4 million tonnes
of hydrogen cyanide (HCN) are produced annually worldwide, of which only about
20% is converted into sodium cyanide (NaCN) and mainly used in the extraction
of precious metals such as gold and silver, and others (e.g. copper). The
remaining 80 % of the hydrogen cyanide (HCN) is used in electroplating, metallurgy,
and in the production of a wide range of chemicals, such as plastics, fire
retardant, cosmetics, dyes, nylon, paints, pharmaceuticals, Plexiglas, rocket
propellant, and road and table salts.
Industrial use of cyanide production
SOURCES OF CYANIDE
Cyanide is a chemical compound possessing highly
toxic potential. There are many sources of cyanide; that exists naturally and
anthropogenically (human made). The two main categories of the cyanide sources
are natural source and the anthropogenic source. Humans are the main
contributors of the cyanide production as compared to the nature.
Natural sources
Cyanide and
chemically related compounds are formed, excreted and degraded in nature by
hundreds of species of bacteria, algae, fungi, plants and insects. Plants that
produce cyanide are cassava, sorghum, alfalfa, bamboo, peach pits, wild cherry,
almond, apricot kernels apple seeds, Lima beans etc. Insects that produce
cyanide are Spiny Dragon, Pleuroloma flavipes, Narcissus annularis etc.
As a result, low
levels of cyanide can appear in naturally occurring surface or groundwater
samples which normally would not be expected to contain it. At least 1,000
species of plants and micro-organisms from 90 families have been shown to
contain one or more of nearly twenty compounds capable of producing cyanide.
About 800 species of higher plants from 70 to 80
families, including agriculturally important species such as the cassava, flax,
sorghum, alfalfa, bamboo, peach, pear, cherry, plum, corn, potato, cotton, almond,
and beans are Cyanogenic (containing a cyanide group in the molecule).
Fungi and bacteria are prevalent producers of
cyanide. In addition to plants and microorganisms, insects have been shown to
produce cyanide. Species of centipedes, millipedes, beetles, moths and
butterflies synthesize and excrete cyanide for defensive purposes. Coffee and
table salt also contain cyanide. Laetrile (an anti-cancer preparation made from
apricot kernels) and sodium nitroprusside (a drug used to reduce high blood
pressure), release cyanide upon metabolism. Non-anthropogenic
sources also include damaged or decaying tissues of plants from the family.
The cyanide content in certain varieties of Lima beans can be as high as 3 mg/g, although values between 0.10 and 0.17 mg/g are
common in Lima beans. Much lower cyanide concentrations in various cereal
grains and cereal products have been reported, ranging from 0.001 to 0.45 μg/g.
Mean cyanide concentrations in soybean products have been found to range from
0.07 to 0.3 μg/g, whereas the mean cyanide concentration in soybean hulls was
1.24 μg/g. Due to the lack of data on cyanide content in total diet samples,
the average daily intake could not be estimated.
The cyanide is also present in many other naturally
occurring things that include Cassava, Wild Cherries, Almonds, Sorghum, Peach
pits, Apricot kernels, Apple seeds, Bamboo shoots etc. Sorghum forage either
green or dry is the main source of livestock feed in dry areas of Pakistan.
However, a lethal risk of hydrogen cyanide (HCN) is associated with this
forage.
A
nitriloside is a naturally occurring compound which upon hydrolysis by a
beta-glucosidase yields a molecule of a non-sugar, or aglycone, a molecule of
free hydrogen cyanide.
The
metabolism of all the higher animals and most of the invertebrates as well,
involves the hydrolysis of plant-derived nitrilosides ingested in the plant
components of the diet. This hydrolysis is produced by betaglucosidase
occurring in the gastro-intestinal tract and produced in various tissues of the
animal. The enzyme occurring in the intestinal tract is produced by various
bacteria or microflora. When the enzyme so produced or that enzyme existing in
the organs acts to hydrolyse the nitrilosides to free HCN, sugar and a
non-sugar moiety the CN [cyanide] ion released is detoxified or converted by an
enzyme normally occurring in the organism and known as rhodanese or thiosulfate
transulfurase. The product of such conversion is thiocyanate, a compound found
in the tissues of all vertebrates, many invertebrates and a number of plants.
Anthropogenic sources
Anthropogenic (of human origin) sources are
responsible for much of the cyanide in the environment. Cyanide has been used
worldwide in the extraction of gold and silver mainly. The major cyanide
releases to water are discharges from metal-finishing industries, iron and
steel mills, electroplating and organic chemical industries plastics and
pharmaceuticals. These industries discharge large quantities of cyanide as in
the form of liquid waste. Effluents from the cyanidation process used in
precious metal extraction contain high amounts of cyanide. Vehicle exhaust and
biomass burning are major sources of cyanide released into the air. The major
sources of simple and complex cyanide releases to soil appear to be from the
disposal of cyanide wastes in landfills and the use of cyanide-containing road
salts.
Cyanogen
chloride is formed in drinking water from reaction of humic substances with
chloramine produced during chlorination. Thiocyanate is released to water
primarily from discharges of industrial waste waters from coal processing and
extraction of gold and silver; the thiocyanate is formed from the reaction of sulphur
donors that are present in coal and crushed rock with the cyanide that is used
in the processing of these materials. Thiocyanate is also found in mining waste
waters where it results from the interaction of the cyanide anion (CN–)
with sulphur. Releases of thiocyanate to soil result from anthropogenic and
natural sources. Anthropogenic releases occur primarily from direct application
in herbicidal formulations and from disposal as by-products from industrial
processes. The mean cyanide
concentration in most surface waters is not greater than 3.5 μg/L.
WHAT HAPPENS TO CYANIDE WHEN
RELEASED TO THE ENVIRONMENT?
Cyanide produce a wide variety of new compounds. Such compounds are often categorized
as simple cyanide compounds, cyanide complexes, and cyanide-related compounds.
The following sections focus on cyanide breakdown compounds most commonly found
at mine sites.
Simple
Cyanide Compounds: Simple cyanide compounds consist of only a single metal ion
in combination with CN. Simple cyanide compounds include sodium cyanide,
potassium cyanide, and calcium cyanide—all of which are readily soluble. Some
simple cyanide compounds are insoluble.
Metal-Cyanide
Complexes: Cyanide complexes are compounds of cyanide bound together with
numerous other organic and inorganic compounds. Only the metal-cyanide,
complexes are compounds.
Cyanides are not persistent in water or soil.
Cyanides may accumulate in bottom sediments, but residues are generally as low
as ˂1 mg/kg even near polluting sources. Majority of an accidental release of
cyanide is volatilized to the atmosphere where it is quickly diluted and
degraded by ultra violet. Other factors, such as biological oxidation,
precipitation and the effects of sunlight also contribute to cyanide
degradation. Cyanide is released into air mainly as hydrogen cyanide gas and,
to a lesser extent, as particulate cyanides. Hydrogen cyanide can potentially
be transported over long distances before reacting with photo chemically
generated hydroxyl radicals. The residence time of hydrogen cyanide in the
atmosphere has been estimated to be approximately 2.5 years, with a range of
1.3–5.0 years, depending on the hydroxyl radical concentration. Neither
photolysis nor deposition by rainwater is expected to be a significant removal
mechanism. Only 2% of the tropospheric hydrogen cyanide is expected to be
transported to the stratosphere.
In water, cyanide occurs most commonly as hydrogen
cyanide. Hydrogen cyanide is expected to be removed from water primarily by
volatilization. Cyanide may also be removed by aerobic or anaerobic
At soil surfaces, volatilization of hydrogen cyanide
is a significant loss mechanism for cyanides. In subsurface soil, cyanide at
low concentrations would probably biodegrade under both aerobic and anaerobic
conditions. In case where cyanide levels are toxic to microorganisms (i.e.,
landfills, spills), the concentrations of water-soluble cyanides may be
sufficiently high to leach into groundwater.
When metal-cyanide complexes are formed and released
into the near-surface environment, they begin to decompose at varying rates,
some quickly, others quite slowly. This breakdown releases cyanide into the
soil or water, generally at relatively low concentrations. Those complexes that
most readily decompose are referred to as weak complexes and those most
resistant to decomposition are called strong complexes. Examples of weak
cyanide complexes include zinc and cadmium cyanides. Moderately strong
complexes include copper, nickel, and silver cyanides. And strong complexes
include iron, cobalt, and gold cyanides.









Comments
Post a Comment